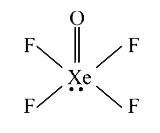
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Number of lone pairs (s) in XeOF(4) is/are
Text Solution
|
- Among the following molecules, (i)XeO(3)(ii)XeOF(4)(iii)XeF(6) those h...
Text Solution
|
- Number of the pairs (s) in XeOF(4) is/are
Text Solution
|
- In XeOF(2) number of lone pairs on cetral atom is 'a' and number of bo...
Text Solution
|
- The type of hybridisation and number of lone pair(s) of electrons of X...
Text Solution
|
- Number of sigma bonds , pi bonds and lone pair on Xe form of XeOF(4)
Text Solution
|
- XeOF(4) में एकल इलेक्ट्रॉन युग्मों की कुल संख्या है :
Text Solution
|
- Among the following molecules (i) XeO(3) (ii) XeOF(4) (iii) XeF(6) tho...
Text Solution
|
- How many lone pairs are there at xenon in XeOF(4) ?
Text Solution
|