Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
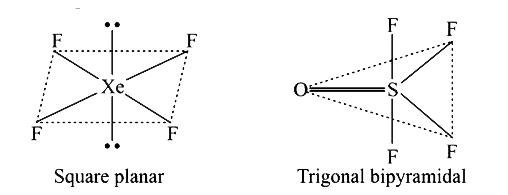
- Draw the shape of XeF(4) and OSF(4) according to VSEPR theory. Show th...
Text Solution
|
- Draw the shape of XeF(4) and OSF(4) according to VSEPR theory Show the...
Text Solution
|
- Write the sum of lone pairs of electron on central atom of SF(4), CF(4...
Text Solution
|
- Draw the shape of XeF(4) and OSF(4) according to VSEPR theory. Show th...
Text Solution
|
- According to VSEPR theory, the most probable shape of the molecule hav...
Text Solution
|
- Using VSEPR thory, draw the molecular structures of OSF(4) and XeF(4)...
Text Solution
|
- Explain the shape of XeF(4) on the basis of VSEPR theory.
Text Solution
|
- The number of lone pair of electrons on the central atom of SF(4),CF(4...
Text Solution
|
- Write the geometry of XeF(4) and SOF(4) and clearly indicate the posit...
Text Solution
|