Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
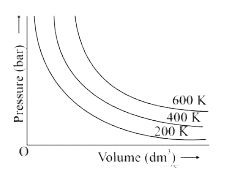
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- Can the temperature of a gas increased keeping its pressure and volume...
Text Solution
|
- If the temperature of a gas is increased by 1K at constant pressure it...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
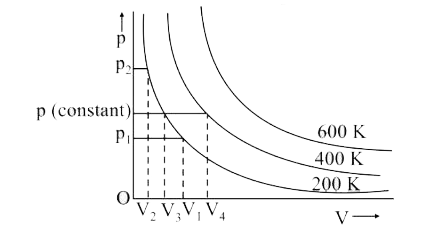
- A graph is plotted between pressure and volume at different temperatur...
Text Solution
|
- क्या किसी गैस का दाब उसके ताप तथा आयतन दोनों को स्थिर रखकर परिवर्तित क...
Text Solution
|
- How does the volume of a definite mass of a gas change with pressure a...
Text Solution
|