Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
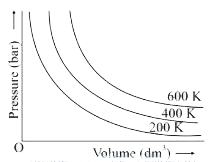
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- The molar specific heat capacity at constant volume (C(V)) for an idea...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- The pressure of a gas varies directly as the remperature when volume ...
Text Solution
|
- 700 मिमी दाब पर किसी गैस का आयतन 200 मिली है। किस दाब पर इसका आयतन 40...
Text Solution
|
- What happens to the pressure of a sample of helium gas if the temperat...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
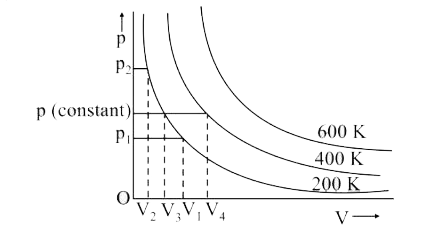
- A graph is plotted between pressure and volume at different temperatur...
Text Solution
|