Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
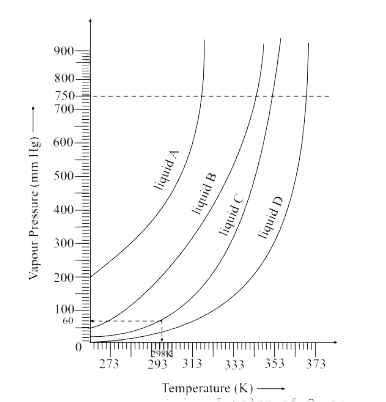
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- Normal boiling point of a liquid is that temperature which vapour pres...
Text Solution
|
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- The variation of vapour of different liquids with temperature is shown...
Text Solution
|
- The vapour pressure of a liquid in a closed container depends on: (1) ...
Text Solution
|
- In the case of immiscible liquids, the addition of one liquid to anoth...
Text Solution
|
- The vapour pressure of a liquid in a closed vessel
Text Solution
|
- The vapour pressure at equilibrium of a liquid in a closed vesse...
Text Solution
|
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|