Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
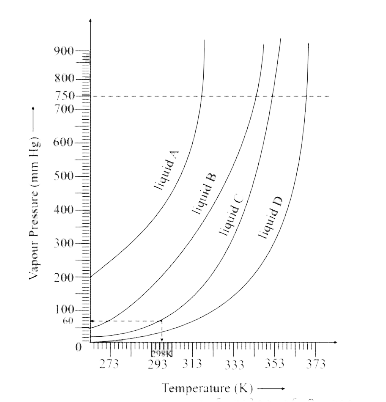
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- At 20^@C, the vapour pressure of pure liquid A is 22 mm Hg and that of...
Text Solution
|
- The vapour pressure of high b.pt. liquids is……..then the vapour pressu...
Text Solution
|
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- Assertion (A) The temperature at which vapour pressure of a liquid is ...
Text Solution
|
- The variation of vapour of different liquids with temperature is shown...
Text Solution
|
- Assertion (A) : The temperature at which vapour pressure of a liquid i...
Text Solution
|
- Assertion : At high altitudes , liquids boil at lower temperatures in ...
Text Solution
|
- Assertion : At high altitudes liquids boil at lower temperaturers in c...
Text Solution
|