Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
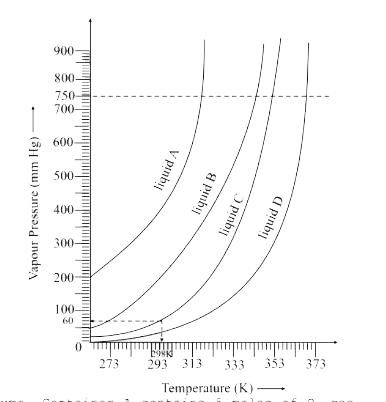
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- The variation of vapour of different liquids with temperature is shown...
Text Solution
|
- In a pressure cooker, cooking is faster because the increases in vapou...
Text Solution
|
- प्रेशर कुकर के उपयोग से खाना जल्दी पकता है, क्यों ?
Text Solution
|
- Explain why does food get quickly cooked in a pressure cooker .
Text Solution
|
- Why pressure cooker is used for cooking food on hills?
Text Solution
|
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- The variation of vapour pressure of different liquids with temperature...
Text Solution
|
- Why are pressure cookers used for cooking on hills?
Text Solution
|