A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
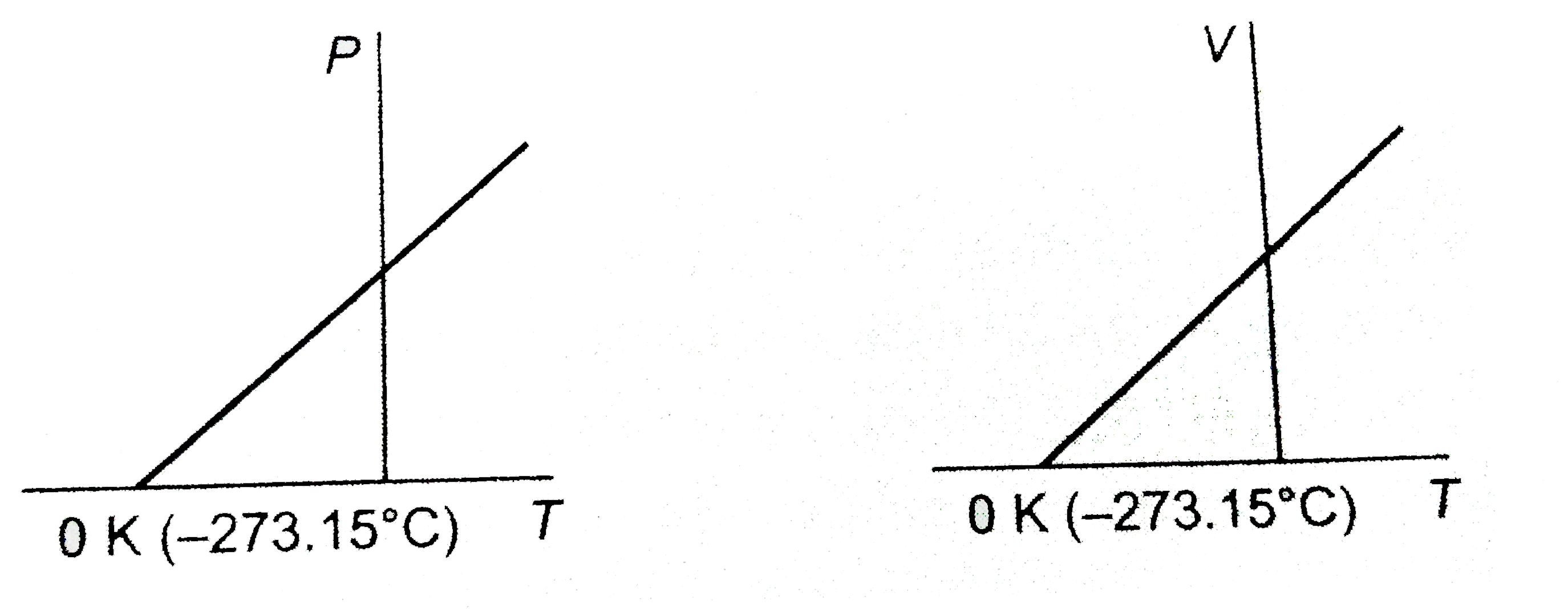
- What conclusion would you draw from the following graphs for an ideal ...
Text Solution
|
- A graph plotted between log t(50%) vs log concentration is a straight ...
Text Solution
|
- A graph plotted between log t(50%) vs log concentration is a straight ...
Text Solution
|
- What conclusion would you draw from the following graphs for an ideal ...
Text Solution
|
- A graph plotted between log t(50%) vs log concentration in a straight ...
Text Solution
|
- What conclusion can you draw about the velocity of a body from the dis...
Text Solution
|
- What conclusion can you draw about the speed of a body from the follow...
Text Solution
|
- What conclusion can you draw about the acceleration of a body from the...
Text Solution
|
- नाभिक की बंधन ऊर्जा से आप क्या समझते हैं? नाभिकों की प्रति न्यूक्लिऑन...
Text Solution
|