Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
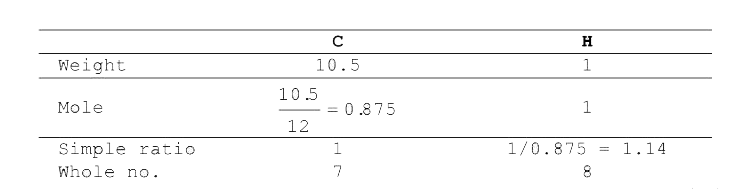
- A hydrocarbon contains 10.5 g of carbon per gram of hydrogen. 1 L of v...
Text Solution
|
- A hydrocarbon contains 10.5 g of carbon per gram of hydrogen. 1 L of v...
Text Solution
|
- A hydrogen contains 10.5 g of carbon per gram of hydrogen. If 1 L of t...
Text Solution
|
- A hydrocarbon contains 10.5 gm carbon and 1 gm hydrogen. Its 2.4 gm ha...
Text Solution
|
- A hydrocarbon contains 10.5 gm carbon and 1gm hydrogen. Its 2.4 gm has...
Text Solution
|
- एक हाइड्रोकार्बन में 10.5 ग्राम कार्बन प्रति ग्राम हाइड्रोजन उपस्थित ह...
Text Solution
|
- किसी हाइड्रोकार्बन में प्रति ग्राम हाइड्रोजन में 9 ग्राम कार्बन उपस्थि...
Text Solution
|
- A hydrocarbon contains 10.5 g of carbon and 1g of hydrogen. The weight...
Text Solution
|
- A hydrocarbon contains 10.5g of carbon per 1g of hydrogen At a tempera...
Text Solution
|