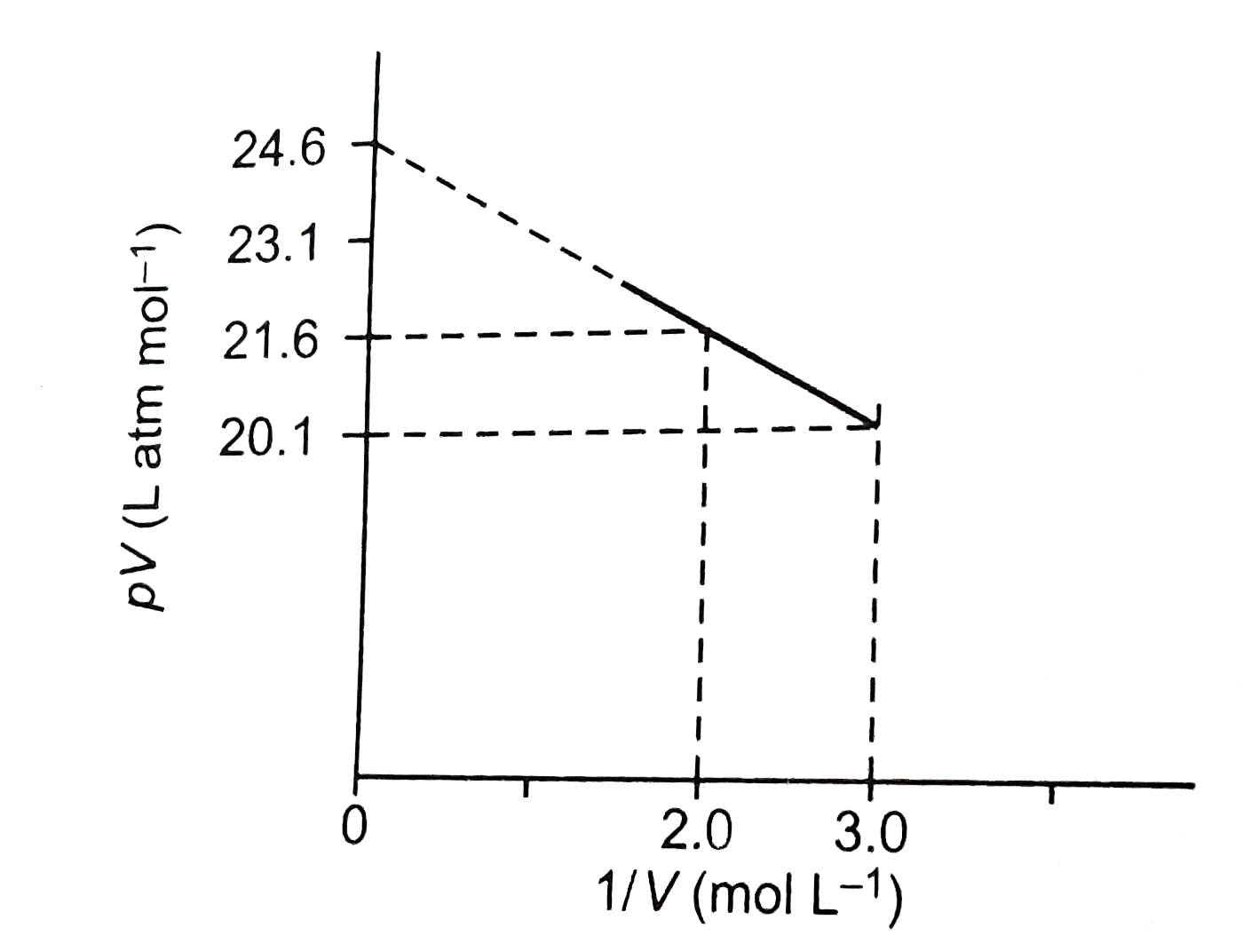
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For one mole of a van der Waals' gas when b=0 and T=300K, the pV vs 1/...
Text Solution
|
- The compression factor (compressibility factor) for 1 mol of a van der...
Text Solution
|
- The compression factor (compressibility factor) for 1 mol of a van der...
Text Solution
|
- For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//...
Text Solution
|
- For one mole of a van der Waals' gas when b=0 and T=300K, the pV vs 1/...
Text Solution
|
- The third virial coefficient of a real gas 2xx10^(-2) (L//"mol")^(2). ...
Text Solution
|
- For one mole of a van der Waal's gas when b = 0 and T = 300K, the PV u...
Text Solution
|
- The compression factor (compressibility factor) for one mole of a van ...
Text Solution
|
- Calculate the critical constants of a gas whose van der Waals constant...
Text Solution
|