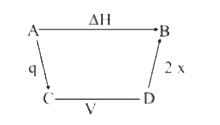
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A hypothetical reaction Ato2B, proceeds through following sequence of ...
Text Solution
|
- A hypothetical reaction, A rarr 2B , proceeds via following sequence o...
Text Solution
|
- Given these reactions : Ato2B DeltaH=40KJ BtoCDeltaH=-50KJ 2CtoD Delta...
Text Solution
|
- Consider the following process :- Ato2B,DeltaH=+150 KJ 3Bto2C+D,De...
Text Solution
|
- The reaction A to B , DeltaH=+24 kJ//"mole" . For the reaction B to C ...
Text Solution
|
- In the reaction C+2SrarrCS(2)+DeltaH, DeltaH is the
Text Solution
|
- 300K ताप पर अभिक्रिया A+b to C+D के लिए DeltaH=350kJ mol^(-1) और Delta...
Text Solution
|
- A hypothetical reaction Ato2B, proceeds through following sequence of ...
Text Solution
|
- A hypothetical reaction, A rarr 2 B proceeds through following sequenc...
Text Solution
|