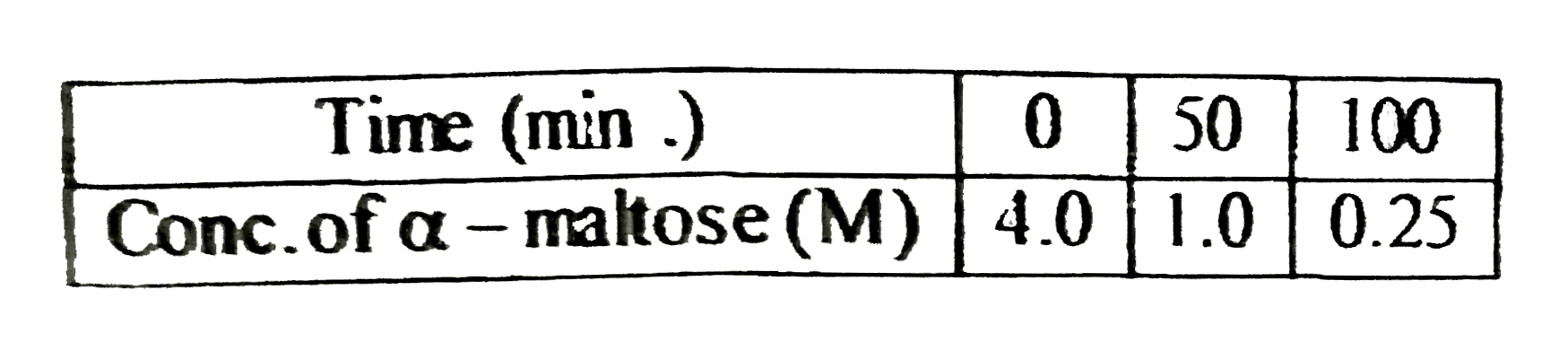A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- alpha- maltose (C(12)H(22)O(11)) can be hydrolysed to glucose (C(6)H(1...
Text Solution
|
- The inversion of cane sugar is represented by C(12)H(22)O(11) + H(2)...
Text Solution
|
- alpha- maltose (C(12)H(22)O(11)) can be hydrolysed to glucose (C(6)H(1...
Text Solution
|
- The inversion of cane sugar is represented by C(12)H(22)O(11) + H(2)...
Text Solution
|
- अभिक्रिया की कोटि को समझाते हुए निम्न अभिक्रिया की कोटि कारण सहि...
Text Solution
|
- सुक्रोस के प्रतिलोमन को निम्न प्रकार व्यक्त किया जा सकता है- C(12)H(...
Text Solution
|
- कथन- गन्ने की शर्करा का प्रतिलोमन C(12)H(22)O(11) + H(2)O overset(H^...
Text Solution
|
- उन एन्जाइमों के नाम लिखिए, जो निम्नलिखित परिवर्तन करते हैं- (a) C(12...
Text Solution
|
- Which if the following enzyme catelyst (X) is used in the conversion o...
Text Solution
|