A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
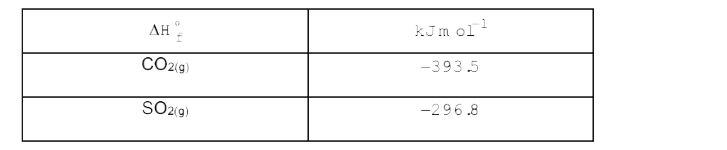
- The combustion of 0.2 mol of liquid carbon disulphide CS2 to give CO2(...
Text Solution
|
- The combustion of 0.200 mol of liquid carbon disulphide, CS(2) to give...
Text Solution
|
- The standard heat of formation of carbon disulphide (l) given that sta...
Text Solution
|
- Given C(2)H(2)(g)+H(2)(g)rarrC(2)H(4)(g): DeltaH^(@)=-175 " kJ mol"^(-...
Text Solution
|
- Calculate the standard heat of formation of carbon disulphide (l). Giv...
Text Solution
|
- The standard enthalpies of combustion of C(6)H(6(l)),C(("graphite"))an...
Text Solution
|
- Calculate the standard heat of formation of carbon disulphide (l), giv...
Text Solution
|
- At 25^(@)C and 1 atm, heat of formation of C(2)H(4) (g), CO(2)(g) and ...
Text Solution
|
- The combustion of 0.2 mol of liquid carbon disulphide CS2 to give CO2(...
Text Solution
|