A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
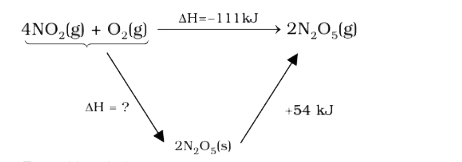
- Consider the reaction, 4NO(2)(g)+O(2)(g)rarr2N(2)O(5)(g),Delta(r )H=...
Text Solution
|
- For the reaction, 2N(2)O(5) (g) rarr 4NO(2)(g) + O(2)(g), the rate o...
Text Solution
|
- Consider the reaction, 4NO(2)(g)+O(2)(g)rarr2N(2)O(5)(g),Delta(r )H=...
Text Solution
|
- Consider folllowing reaction at 300 K : N(2)O(5)(g)hArrN(2)O(5)(g)+O(2...
Text Solution
|
- The Delta(f) H^(@) (N(2)O(5), g) in kJ/mol on the basic of the followi...
Text Solution
|
- Consider the reaction 4 NO(2)(g) +O(2)(g)rarr 2N(2)O(5)(g), Delta(r)H ...
Text Solution
|
- Consider the reaction 2N(2) O(5(g) ) to 4NO(2(g)) +O(2(g)) in liquid b...
Text Solution
|
- Consider the reaction : 4 NO(2 (g)) + O(2 (g)) to 2 N(2)O(5 (g)) , Del...
Text Solution
|
- Comment on the thermodynamic stability of NO(g), given, (i) 1/2 N(2)(g...
Text Solution
|