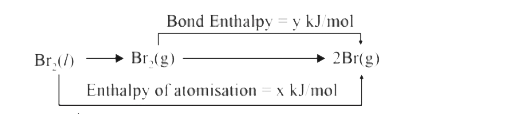A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If enthalpy of atomisatio for Br((2(1))) is xKJ//mol, the relation bet...
Text Solution
|
- DeltaU^(Θ) of combustion of methane is -XkJ mol^(-1). The value of Del...
Text Solution
|
- Liquid bromine boils at 332.7.K Estimate the enthalpy of formation of ...
Text Solution
|
- DeltaU^(@) of combustoin of methane is -XKJ mol ^(-1) The value of Del...
Text Solution
|
- The enthalpy and entropy change for the reaction Br(2)(l)+Cl(2)(g)rarr...
Text Solution
|
- The enthalpy and entropy change for the reaction: Br(2)(l) + Cl(2)(g) ...
Text Solution
|
- If enthalpy of atomisation for Br((2(l))) is xKJ//mol bond enthalpy fo...
Text Solution
|
- Bond enthalpy of bromine is 194KJmol^(-1) .If enthalpy of vapourisatio...
Text Solution
|
- Bond enthalpy of bromine is 194KJmol^(-1) .If enthalpy of vapourisatio...
Text Solution
|