Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
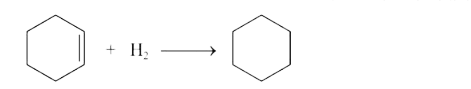
- The standard ethelpy of combustion at 25^(@)C of hydrogen, cyclohexene...
Text Solution
|
- The standard enthalpy of combustion at 25^(@)C of hydrogen, cyclohexen...
Text Solution
|
- The enthalpy of combustion at 25^(@)C of H(2)(g), cyclohexane and cycl...
Text Solution
|
- The standard ethelpy of combustion at 25^(@)C of hydrogen, cyclohexene...
Text Solution
|
- The standard ethelpy of combustion at 25^(@)C of hydrogen, cyclohexene...
Text Solution
|
- हाईडोजन, साइक्लोहेक्सिजन (C6H10) एव साइक्लोहेक्सेन (C6H12) की 25^@C प...
Text Solution
|
- At 25^(@)C , standard enthalpy of combustion of combustion of hydrogen...
Text Solution
|
- 25”^(@)C पर हाइड्रोजन, साइक्लोहेक्सीन (C6H10) तथा साइक्लोहेक्सेन (C6H1...
Text Solution
|
- 25^(@)C पर H(2), साइक्लोहेक्सीन (C(6)H(10)) और साइक्लोहेक्सेन (C(6)H(1...
Text Solution
|