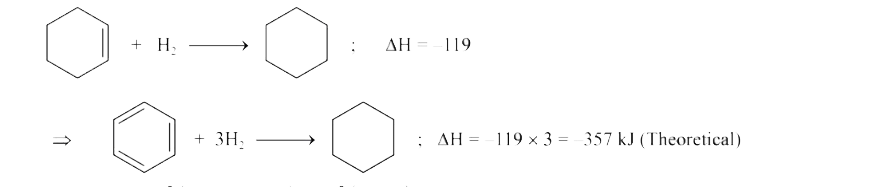
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The standard molar enthalpies of formation of cyclohexane (I) and benz...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (l) and benz...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (l) and benz...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (I) and benz...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5 kJ mol^(-1) . I...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane(l ) and benz...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5kJ mol^(-1). If ...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexane is -119.5 kj*mol ^(-1) . ...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (l) and benz...
Text Solution
|