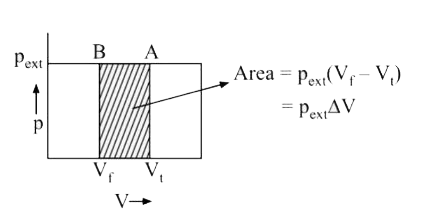
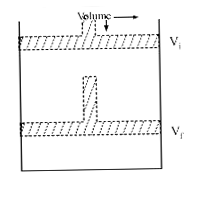Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- What will be the work done on an ideal gas enclosed in a cylinder, whe...
Text Solution
|
- If the boundary of system moves by an infinitesimal amount, the work i...
Text Solution
|
- What will be the work done on an ideal gas enclosed in a cylinder, whe...
Text Solution
|
- How will you calculate work done on an ideal gas in a compression, whe...
Text Solution
|
- How will you calculate work done on an ideal gas in a compression, whe...
Text Solution
|
- What would be the sign convention for work done on a system for a cyli...
Text Solution
|
- How will you calculate work done on an ideal gas in a compression, whe...
Text Solution
|
- What will be the work done on an ideal gas enclosed in a cylinder, whe...
Text Solution
|
- Work done on a ideal gas in a cylinder when it is compressed by an ext...
Text Solution
|