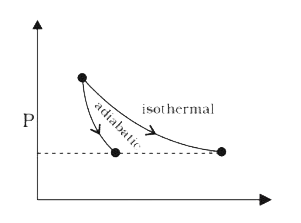
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If a certain mass of gas is made to undergo separately adiabatic and i...
Text Solution
|
- A sample of ideal gas undergoes isothermal expansion in a reversible m...
Text Solution
|
- If a certain mass of gas is made to undergo separately adiabatic and i...
Text Solution
|
- StatementI: Work done by a gas in isothermal expansion is more than th...
Text Solution
|
- Four sample of ideal gas containig same moles and intially at same ...
Text Solution
|
- For an ideal gas, the isothermal free expansion and adiabatic free exp...
Text Solution
|
- A : Work done by a gas in isothermal expension is more than the work d...
Text Solution
|
- Two identical samples of a gas expands so that volume is doubled. The ...
Text Solution
|
- Two gases have the same initial pressure, volume and temperature. They...
Text Solution
|