A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
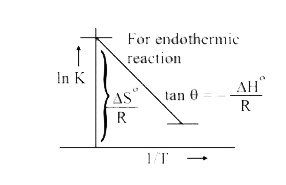
- A plot of ln k against (1)/(T) (abscissa) is expected to be a straight...
Text Solution
|
- A plot of log (x/m) against log P for the adsorption of a gas on a slo...
Text Solution
|
- In accordance to Arrhenius equation, the plot of log k against (1)/(T)...
Text Solution
|
- For a first order reaction, the plot of log k against 1/T is a straigh...
Text Solution
|
- A plot of log (a-x) against time 't' is a straight line. This indicate...
Text Solution
|
- A plot of ln k against (1)/(T) (abscissa) is expected to be a straight...
Text Solution
|
- For the order reaction , plot of log (10) (a-x) against time t is a st...
Text Solution
|
- For a first order reaction the plot of ‘t’ against log c gives a strai...
Text Solution
|
- A plot of In K against 1/T (abscissa) is expected to be a straight lin...
Text Solution
|