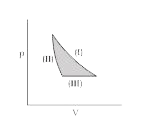
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following cyclic process. I. Isothermal , II. Adiaba...
Text Solution
|
- The internal energy of a system remains constant when it undergoes (i)...
Text Solution
|
- The molar heat capacity for an ideal gas (i) Is zero for an adiabatic ...
Text Solution
|
- Isothermal Process and Adiabatic Process.
Text Solution
|
- Work done in an isothermal process at constant pressure is
Text Solution
|
- Among the following processes identify those in which the change in in...
Text Solution
|
- Consider the following cyclic process. I. Isothermal , II. Adiabatic ,...
Text Solution
|
- Explain (i) Reversible and irreversible process (ii) Isothermal an...
Text Solution
|
- Define the following terms : (i) System (ii) Isothermal and adiaba...
Text Solution
|