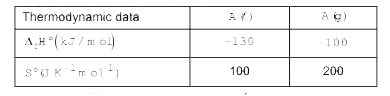A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assuming DeltaH^(@) and S^(@) do not change with temperature. Calculat...
Text Solution
|
- Assuming DeltaH^(@) and S^(@) do not change with temperature. Calculat...
Text Solution
|
- The boiling point of a liquid is 68.9^(@)C . Calculate its aproximate ...
Text Solution
|
- The temperature of a liquid at its boiling point does not change. Wha...
Text Solution
|
- क्वथनांक से कम तापमान पर द्रव के वाष्पन में परिवर्तन की प्रक्रिया को क...
Text Solution
|
- Assuming DeltaH^(@) and S^(@) do not change with temperature. Calculat...
Text Solution
|
- द्रवों के क्वथनांक कमरे के ताप पर होते है
Text Solution
|
- Assuming Delta H^(0) and Delta S^(0) do not change with temperature th...
Text Solution
|
- The phenomenon of changing of a liquid into vapours at any temperature...
Text Solution
|