A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
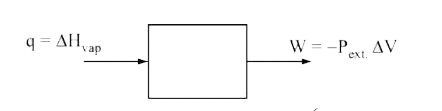
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|
- Assuming that water vapour in an ideal gas, the internal energy change...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy (Delta...
Text Solution
|
- If water vapour is assumed to be a perfect gas, molar enthalpy change ...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|
- If water vapour is assumed to be a perfect gas, molar enthalpy change ...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|