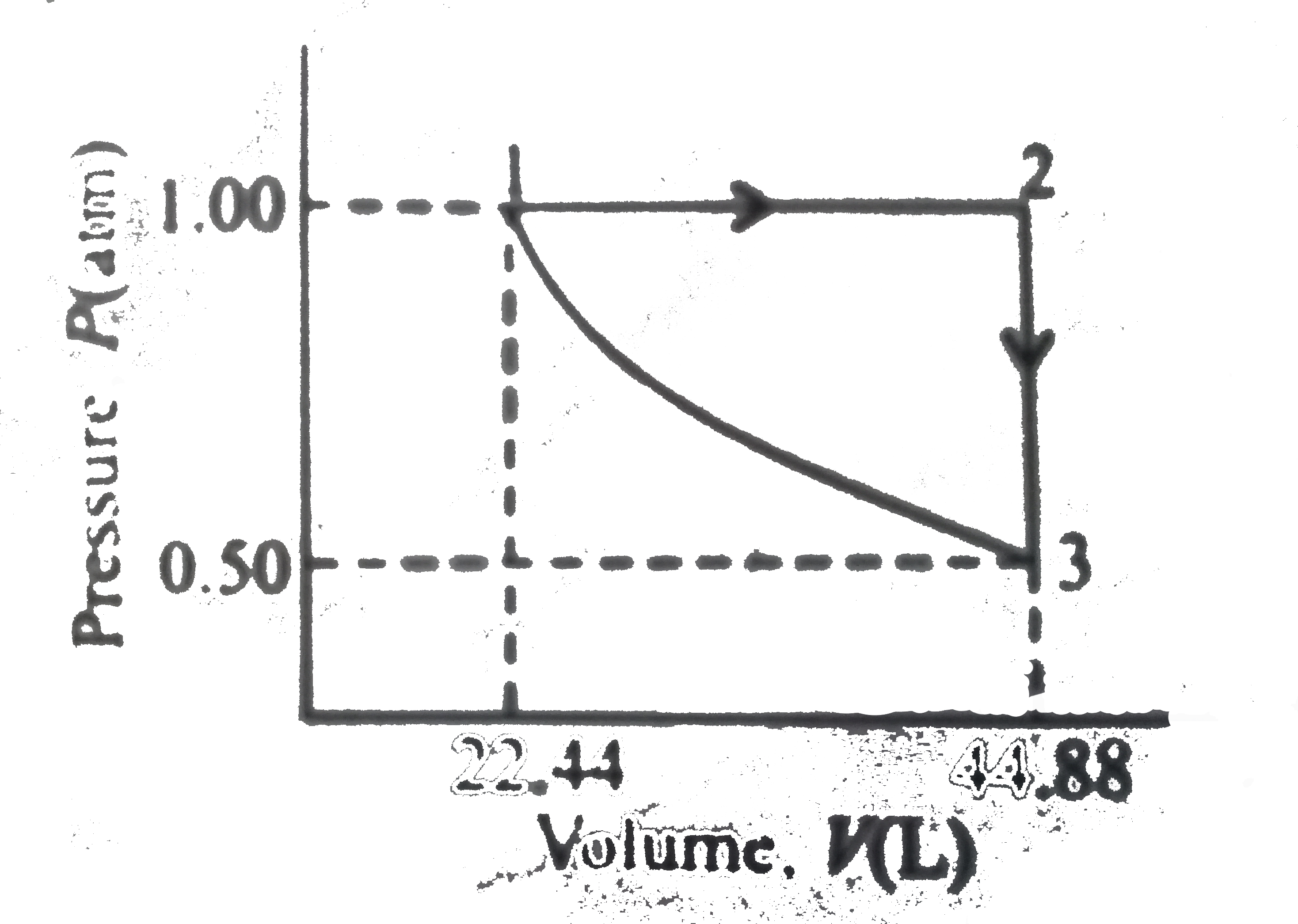
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- One mole of a monoatomic ideal gas is taken through the cycle ABCDA as...
Text Solution
|
- 1mol of a mono-atomic gas is subjected to following cyclic process: ...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A mono-atomic ideal gas is compressed form volume V to V//2 through va...
Text Solution
|
- Ideal mono-atomic gas is taken through process such that dQ = 3dU. The...
Text Solution
|