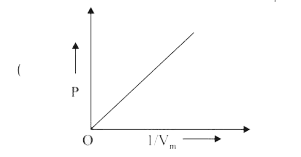
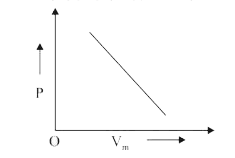
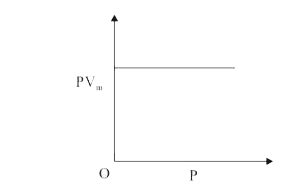
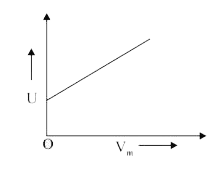
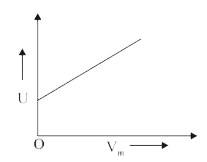
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- For isothermal expansion of an ideal gas, the correct combination of t...
Text Solution
|
- एक आदर्श गैस के समतापी प्रसार में-
Text Solution
|
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- एक आदर्श गैस के समतापी प्रसार में-
Text Solution
|
- For an isothermal expansion of an ideal gas, the correct combination o...
Text Solution
|
- For the isothermal expansion of an ideal gas
Text Solution
|
- For isothermal expansion of an ideal gas, the correct combination of t...
Text Solution
|