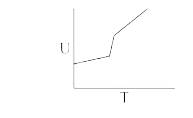
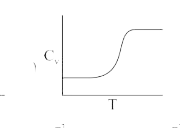
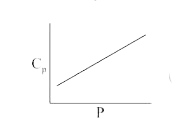
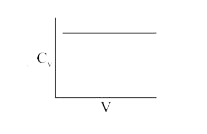
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- for a diatomic ideal gas in a closed system , which of the follo...
Text Solution
|
- Find the efficeincy of the thermodynamic cycle shown in figure for an ...
Text Solution
|
- An ideal gas is subjected to various changes which are plotted as foll...
Text Solution
|
- for a diatomic ideal gas in a closed system , which of the follo...
Text Solution
|
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- Which of the following relations does an ideal gas follow in an adiaba...
Text Solution
|
- Which one of the following equations does not correctly represent the ...
Text Solution
|
- निम्न में से कौन -सा समीकरण थर्मोडायनामिक्स के प्रथम सिध्दांत को दिए ग...
Text Solution
|