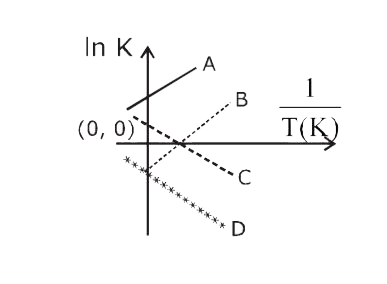
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following lines correctly show the temperature dependence...
Text Solution
|
- Which of the following lines correctly show the temperatrue dependence...
Text Solution
|
- For an exothermic reaction, what happens to the equilibrium constant i...
Text Solution
|
- Which of the following lines correctly show the temperature dependence...
Text Solution
|
- Which of the following lines correctly show the temperature dependence...
Text Solution
|
- On increasing temperature, the equilibrium constant of exothermic and ...
Text Solution
|
- Which of the following lines correctly show the temperature dependence...
Text Solution
|
- For an exothermic reaction , temperature increase by 10^@C , the equil...
Text Solution
|
- ऊष्माक्षेपी अभिक्रिया के लिये निम्नलिखित रेखाओं में से कौन-सी रेखाओं द...
Text Solution
|