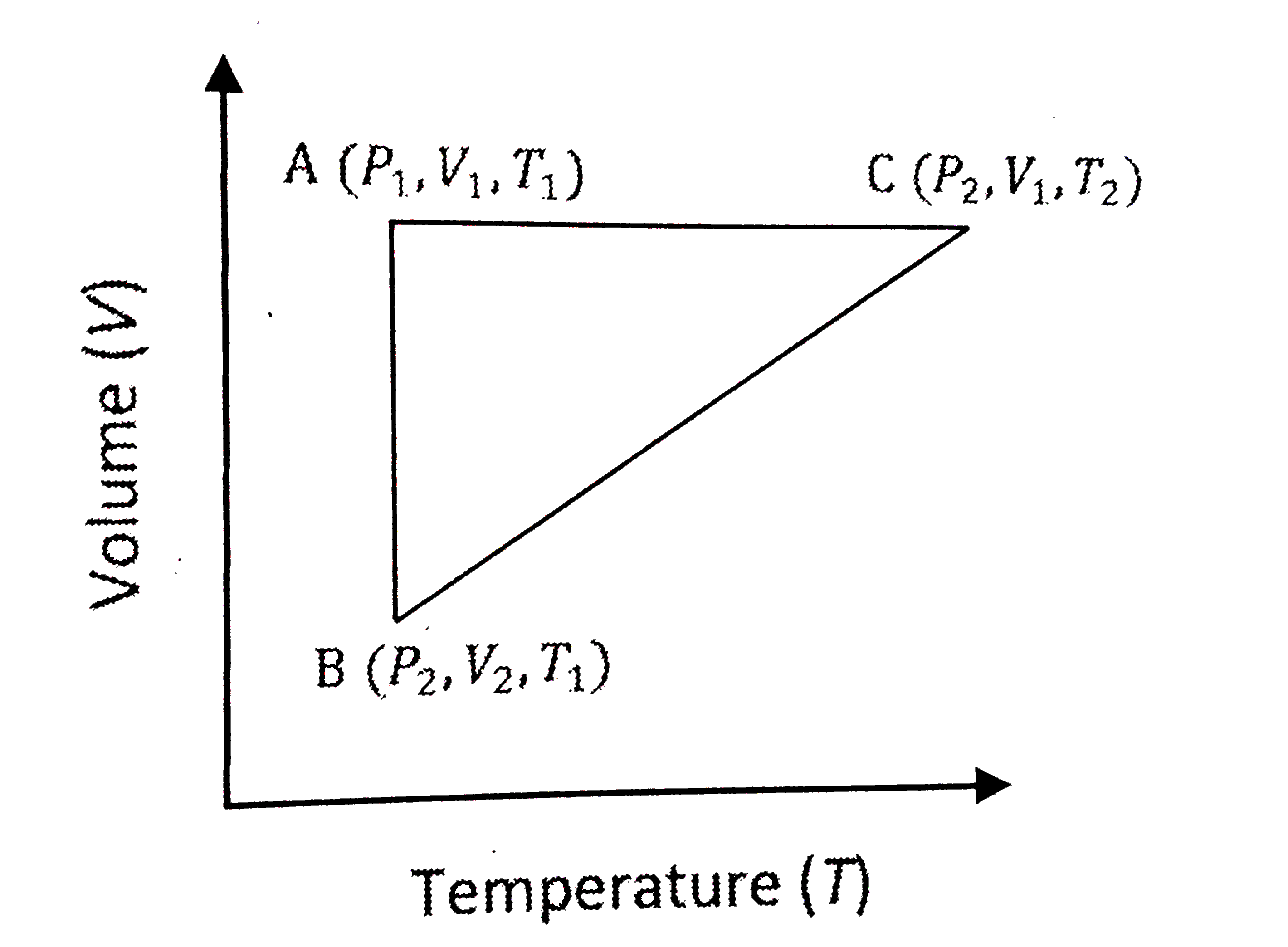
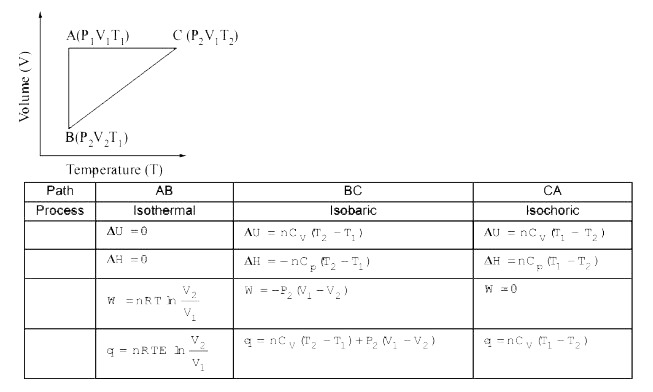
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A reversible cyclic process for an ideal gas is shown below. Here, P, ...
Text Solution
|
- The cyclic process for 1 mole of an ideal gas is shown in the V-T diag...
Text Solution
|
- Density (rho) versus internal energy (U) graph of a gas is as shown in...
Text Solution
|
- A reversible cyclic process for an ideal gas is shown below. Here, P, ...
Text Solution
|
- If the internal energy of an ideal gas U and volume V are doubled, the...
Text Solution
|
- यदि एक बंद आवर्त ( चक्रीय ) प्रक्रिया में Q,E और W क्रमशः डाली गई ऊष्म...
Text Solution
|
- An ideal gas (whose Cp/Cv=lambda, and internal energy U at absolute z...
Text Solution
|
- एक आदर्श गैस के लिए एक उत्क्रमणीय चक्रीय नीचे आकृति में दिखाया गया है।...
Text Solution
|
- If Q, E and W denote respectively the heat added, change in internal e...
Text Solution
|