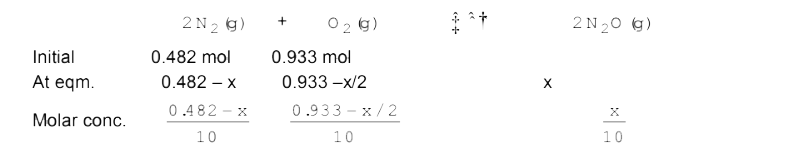Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Reaction between nitrogen and oxygen takes place as following: 2N(2(...
Text Solution
|
- Reaction between nitrogen and oxygen takes place as following: 2N(2(g)...
Text Solution
|
- Consider folllowing reaction at 300 K : N(2)O(5)(g)hArrN(2)O(5)(g)+O(2...
Text Solution
|
- Reaction between nitrogen and oxygen takes place as follows : 2 N(2) (...
Text Solution
|
- N(2) एवं O(2) के मध्य निम्नलिखित अभिक्रिया होती है- यदि 10 L के एक ...
Text Solution
|
- N(2) एवं O(2) के मध्य निम्नलिखित अभिक्रिया होता है - 2N(2)(g)+O2(g)h...
Text Solution
|
- Reaction between N(2) and O(2) takes place as follows: 2N(2)(g)+O(2)(g...
Text Solution
|
- For reaction 2NO(g)hArr N(2)(g)+O(2)(g) degree of dissociation of N(2)...
Text Solution
|
- Reaction between N(2) and O(2-) takes place as follows : 2N(2)(g)+O(2)...
Text Solution
|