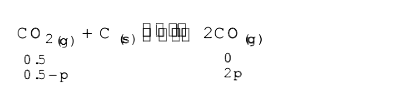A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A vessel at 1000 K contains carbon dioxide with a pressure of 0.5 atm....
Text Solution
|
- A vessel at 1000 K contains carbon dioxide with a pressure of 0.5 atm....
Text Solution
|
- A vessel at 1000 K contains carbon dioxide with a pressure of 0.5 atm....
Text Solution
|
- A vessel at 1000 K contains CO2 with a pressure of 0.5 atm. Some of t...
Text Solution
|
- 1000 K ताप और 0.5 वायुमंडल दाब पर एक बर्तन में कार्बन डाइऑक्साइड गैस भ...
Text Solution
|
- A vessel at 1000 K contains CO(2) with a pressure of 0.5 atm. Some of ...
Text Solution
|
- A vessel at 100K contains CO(2) with a pressure of 0.5 atm. Some of th...
Text Solution
|
- A vessel at 1000K contains CO2 with a pressure of 0.5 atm. Some of the...
Text Solution
|
- A vessel at 1000 K contains CO(2) with a pressure of 0.5 atm. Some of ...
Text Solution
|