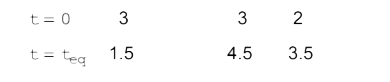A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Three moles of PCl(5), three moles of PCl(3) and two moles of Cl(2) ar...
Text Solution
|
- 1 mole of PCl(5) taken at 5 atm, dissociates into PCl(3) and Cl(2) to...
Text Solution
|
- Three moles of PCl(5) , three moles of PCl(3) and two moles of Cl(2) a...
Text Solution
|
- A quantity of PCl(5) ws heated in a 10 litre vessel at 250^(@)C: PCl(5...
Text Solution
|
- Three moles of PCl(5). three moles of PCl(3) and two moles of Cl(2) ar...
Text Solution
|
- 1*5 moles of PCl(5) are heated at constant temperature in a closed ves...
Text Solution
|
- a' moles of PCl(5) are heated in a closed container to equilibrate PCl...
Text Solution
|
- a' moles of PCl(5) are heated in a closed container to equilibrate PCl...
Text Solution
|
- PCl(5) के 3 मोल, PCl(3) के 3 मोल तथा Cl(2) के 2 मोलों को एक बंद पात्र ...
Text Solution
|