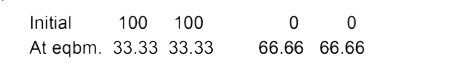A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A + B hArrC + D. If finally the concentrations of A an d B are both eq...
Text Solution
|
- A + B hArrC + D . If finally the concentrations of A an d B are both e...
Text Solution
|
- For the reaction a+brArrc+d, initially concentrations of a and b are e...
Text Solution
|
- For the reaction, A+BhArr C+D, the initial concentration of A and B ar...
Text Solution
|
- A+B hArr C+D is 100. If intially the concentrations of A and B are equ...
Text Solution
|
- For the reaction a+bhArrc+d , initially concentrations of a and b are ...
Text Solution
|
- A+B hArr C+D if intially the concentration A and B are equal but at eq...
Text Solution
|
- A+B hArr C+D . If initially the concentration of A and B are both equa...
Text Solution
|
- अभिक्रिया a + b hArr c+ d के लिए, a व b की प्रारम्भिक सांद्रताएँ ब...
Text Solution
|