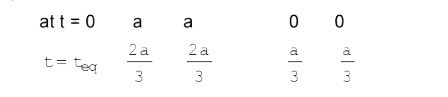A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compounds A and B are mixed in equimolar proportion to form the pr...
Text Solution
|
- When 4 mol of A is mixed with 4 mol of B, 4 mol of C and D are formed ...
Text Solution
|
- The compounds A and B are mixed in equimolar proportion to form the pr...
Text Solution
|
- 4 moles of A are mixed with 4 moles of B. At equilibrium for the racti...
Text Solution
|
- The compounds A and B are mixed in equimolar proportion to form the pr...
Text Solution
|
- योगिक A और B सम्मोलर अनुपात में उत्पाद निर्मित करने के लिए मिश्रित होत...
Text Solution
|
- A+B hArr C+D if intially the concentration A and B are equal but at eq...
Text Solution
|
- The compounds A and B are mixed in equimolar proportion to form the pr...
Text Solution
|
- यदि A harr B (K(c) = 3), B harr C (K(c) = 5), C harr D (K(c) = 2) उपरो...
Text Solution
|