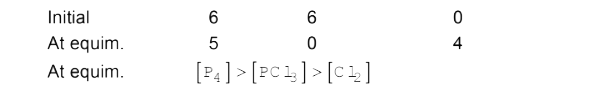A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The equilibrium, P(4)(s) + 6 Cl(2)(g) rarr 4PCl(3) attained by mixing ...
Text Solution
|
- The equilibrium: P(4)(g)+6Cl(2)(g) hArr 4PCl(3)(g) is attained by ...
Text Solution
|
- 4 mole of Sl(2)Cl(4)(g) is introduced innto a 10L vessel. The followin...
Text Solution
|
- The equilibrium P(4) (s) + 6 Cl(2) (g) hArr 4 PCl(3) (g) is attained b...
Text Solution
|
- 2 लीटर के एक बर्तन में अभिकारकों व उत्पाद की साम्य सांद्रताएँ निम्न है...
Text Solution
|
- अभिक्रिया P(4)(s) + 5O(2)(g) ltimplies P(4)O(10)(s) के लिए साम्य सीथरा...
Text Solution
|
- Balance the following chemical equations: P(4(s))+6Cl(2(g))rarr 4PC...
Text Solution
|
- Balance the following chemical equations: P(4(s))+6Cl(2(g))rarr 4PC...
Text Solution
|
- The equilibrium, P(4)(s) + 6 Cl(2)(g) rarr 4PCl(3) attained by mixing ...
Text Solution
|