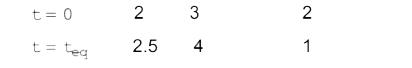A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a reaction A+2B hArr 2C, 2.0 moles of 'A' 3 moles of 'B' and 2.0 m...
Text Solution
|
- For the reaction, A+B hArr 2C, 2 mol of A and 3 mol of B are allowed t...
Text Solution
|
- In a reaction A+2BhArr2C, 2.0 mole of 'A' , 3.0 mole of 'B' and 1 mole...
Text Solution
|
- In a reaction A+2B hArr 2C, 2.0 moles of 'A' 3 moles of 'B' and 2.0 m...
Text Solution
|
- The equilibrium constant of a reaction A + B hArr 2C if the concentrat...
Text Solution
|
- In the reaction A+2B hArr 2C, if 2 moles of A, 3.0 moles of B and 2.0 ...
Text Solution
|
- In a reaction , A+ 2 B hArr 2 C, 2.0 mole of 'A' 3.0 mole of 'B' and 2...
Text Solution
|
- In a reaction, A + 2B hArr 2C, 2.0 mole of A, 3.0 mole of B and 2.0 mo...
Text Solution
|
- For the reaction, A(g)+2B(g)hArr2C(g) one mole of A and 1.5 mol of B a...
Text Solution
|