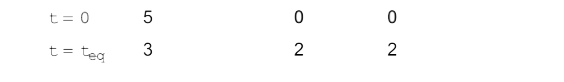A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a 500mL falsk, the degree of dissociation of PCI(5) at equilibrium ...
Text Solution
|
- A quantity of PCI(5) was heated in a 10 llitre vessel at 250^(@)C, PCI...
Text Solution
|
- In a reaction PCI(5)hArrPCI(3) + CI(2) degree of dissociation is 30% ....
Text Solution
|
- 2 moles of PCI(5) was heated in a closed vessel of 2 litre capacity. A...
Text Solution
|
- PCI(5) (g)hArrPCI(3)(g) + CI(2)(g) DeltaG(f)^(circ)[PCI(5)(g)]=-74kcal...
Text Solution
|
- In a 500mL falsk, the degree of dissociation of PCI(5) at equilibrium ...
Text Solution
|
- By dissociation of 4g mol of of PCI(5) produce 0.8 mol of PCI(3) if vo...
Text Solution
|
- Asseration : In the dissociation of PCI(5) at constant pressure and te...
Text Solution
|
- The degree of dissociation of PCI(5) (alpha) obeying the equilibrium, ...
Text Solution
|