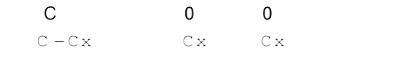A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Phosphorus pentachloride dissociates as follows, ina closed reaction v...
Text Solution
|
- The reaction, PC1(5)hArrPC1(3) + C1(2) is started in a 5 litre contain...
Text Solution
|
- Phosphorus pentachloride dissociates as follows, ina closed reaction v...
Text Solution
|
- Which of the following factor is shifted the reaction PC1(3) + C1(2)hA...
Text Solution
|
- In the following reaction PC1(5)(g)hArrPC1(3)(g) + C1(2)(g) at const...
Text Solution
|
- Suppose the reaction PC1(5(s))hArrPC1(3(s)) + C1(2(g)) is in a closed ...
Text Solution
|
- The degree of dissociation of PC1(5) (alpha) obeying the equilibrium, ...
Text Solution
|
- If dissociation for reaction, PC1(5)hArrPC1(3) + C1(2) is 20% at 1 atm...
Text Solution
|
- Given for the following equilibrium taking place in 1 L flask at 300 K...
Text Solution
|