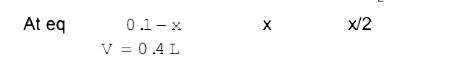A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the percent dissociation of H(2)S(g) if 0.1 mol of H(2)S is ...
Text Solution
|
- Calculate the precentage dissociation of H(2)S(g) if 0.1 mol of H(2)S ...
Text Solution
|
- What is the % dissociation of H(2)S if 1 "mole" of H(2)S is introduced...
Text Solution
|
- Calculate the percent dissociation of H(2)S(g) if 0.1 mol of H(2)S is ...
Text Solution
|
- Calculate K(c) for the reaction: 2H(2)(g)+S(2)(g) hArr 2H(2)S(g) if 1....
Text Solution
|
- An equilibrium mixture for the reaction 2H(2)S(g) hArr 2H(2)(g) + S(...
Text Solution
|
- Calculate the percent dissociation of H(2)S(g) if 0.1 mol of H(2)S is ...
Text Solution
|
- H(2)S(g) के वियोजन की प्रतिशत मात्रा ज्ञात कीजिये, यदि H(2)S के 0.1 मो...
Text Solution
|
- The value of K(p) for the reaction 2H(2)S(g) hArr 2H(2)(g) +S(2)(g) is...
Text Solution
|