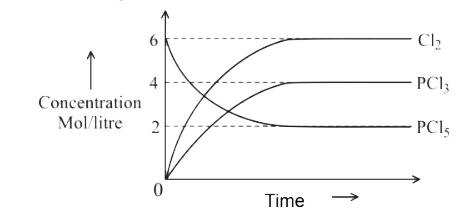
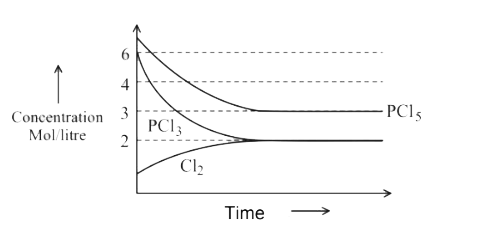
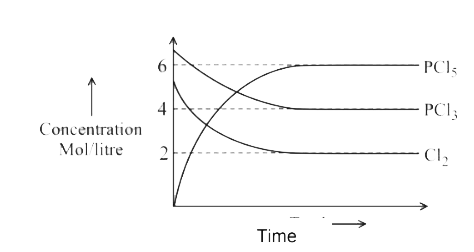
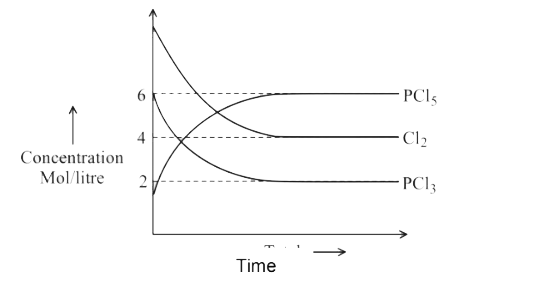
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction PCl(5)(g)hArrPCl(3)(g)+Cl(2)(g) Which of the foll...
Text Solution
|
- For the reaction PCl(5)(g)hArrPCl(3)(g)+Cl(2)(g) Which of the foll...
Text Solution
|
- For the reaction, PCl(3)(g)+Cl(2)(g) hArr PCl(5)(g) , the position of ...
Text Solution
|
- The equilibrium constant (K(p)) for the reaction, PCl(5(g))hArrPCl(3(g...
Text Solution
|
- The equilibrium constant for the following reactions are K(1) and K(2)...
Text Solution
|
- For the reaction Pcl(5(g))hArrPCl(3(g))+Cl(2(g))
Text Solution
|
- At a given temperature the equilibrium constant for the reaction of ...
Text Solution
|
- अभिक्रिया, PCl(5)(g) ltimplies PCl(3)(g) + Cl(2)(g) के लिए साम्य ताप प...
Text Solution
|
- At 27^(@)C, K(p) value for the reversible reaction PCl(5)(g)harrPCl(3)...
Text Solution
|