A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
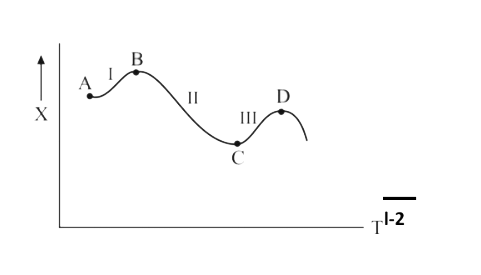
- For the following reaction through stages I, II and III A overset("I")...
Text Solution
|
- For the following reaction through stages I, II and III A overset(I)...
Text Solution
|
- Complete the given table showing different possililities of genotypes ...
Text Solution
|
- In the following sequence : CH(3)CH(2)Cloverset(NaCN)(to) (i) overse...
Text Solution
|
- Considering the following reactions (I,II,III) and give your answer fo...
Text Solution
|
- Considering the following reactions (I,II,III) and give your answer fo...
Text Solution
|
- The major product of the following reaction is : overset((i)NaNO2//H^+...
Text Solution
|
- For the following reaction through stages I, II and III A overset("I")...
Text Solution
|
- Complete the given table showing different possililities of genotypes ...
Text Solution
|