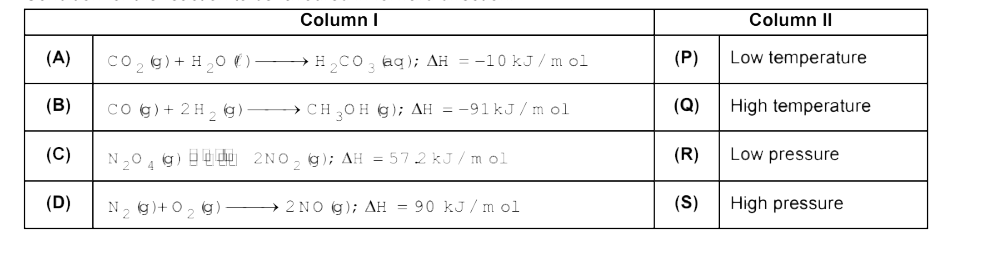
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Match the following : Condition for the reaction to be favoured in for...
Text Solution
|
- Which among the following reactions is favoured in forward direction b...
Text Solution
|
- Which of the following conditions is (are) favourable for the feasibil...
Text Solution
|
- The favourable conditions for a spontaneous reaction are
Text Solution
|
- Among the following reaction which favours forward reaction ?
Text Solution
|
- Consider the reaction equilibrium underset(("Greater volume"))("Ice")h...
Text Solution
|
- Which of the following conditions favour E2 reaction
Text Solution
|
- Match the following : Condition for the reaction to be favoured in for...
Text Solution
|
- Increase in temperature favour the forward reaction in
Text Solution
|