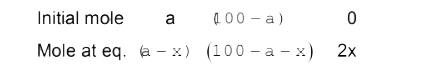Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A sample of air consisting of N(2) and O(2) was heated to 2500 K until...
Text Solution
|
- A sample of air consisting of N(2) and O(2) was heated to 2500 K until...
Text Solution
|
- In the equilibrium constant for N(2)(g) + O(2)(g)hArr2NO(g) is K, the ...
Text Solution
|
- Consider folllowing reaction at 300 K : N(2)O(5)(g)hArrN(2)O(5)(g)+O(2...
Text Solution
|
- If the equilibrium constant for N(2) (g) + O(2)(g) hArr 2NO(g) is K...
Text Solution
|
- A sample of air consisting of N(2) and O(2) was heated to 2500 K until...
Text Solution
|
- If the equilibrium constant for N(2) (g) + O(2)(g) hArr 2NO(g) is K...
Text Solution
|
- If the equilibrium constant for N(2)(g)+O(2)(g) hArr 2NO(g) is K, ...
Text Solution
|
- For reaction 2NO(g)hArr N(2)(g)+O(2)(g) degree of dissociation of N(2)...
Text Solution
|