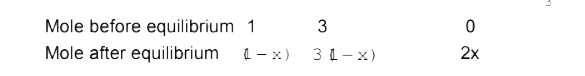Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- At 450^(@)C the equilibrium constant K(p) for the reaction N(2)+3H(2) ...
Text Solution
|
- In a mixture of N(2) and H(2) in the ratio 1:3 at 30 atm and 300^(@)C ...
Text Solution
|
- K(p) for the reaction N(2)+3H(2) hArr 2NH(3) is 1.6xx10^(-4) atm^(-2) ...
Text Solution
|
- At 450^(@)C the equilibrium constant K(p) for the reaction N(2)+3H(2) ...
Text Solution
|
- In a mixture of N(2) and H(2) in the ratio 1:3 at 30 atm and 300^(@)C ...
Text Solution
|
- For N(2)+3H(2)hArr 2NH(3), 1 mole N(2) and 3 mol H(2) are at 4 atm. Eq...
Text Solution
|
- For the equilibrium N(2)+3H(2) hArr 2NH(3) at 298K and 5 atm. Pressure...
Text Solution
|
- Calculate K(p) for the following reaction if partial pressures of NH(3...
Text Solution
|
- Equilibrium constant for the reaction , N(2) + 3 H(2) hArr 2 NH(3) is ...
Text Solution
|