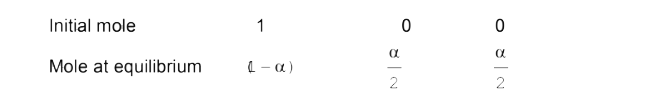Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the dissociation of HI, 20% of HI is dissociated at equilibrium. Ca...
Text Solution
|
- For a reaction 2HI hArr H(2)+I(2) , at equilibrium 7.8 g, 203.2 g , an...
Text Solution
|
- In the dissociation of HI, 20% of HI is dissociated at equilibrium. Ca...
Text Solution
|
- The value of K(p) for dissociation of 2HI hArr H(2)+I(2) is 1.84xx10^(...
Text Solution
|
- When the system 2HI(g) hArr H(2)(g)+I(2)(g) is at equilibrium, inert g...
Text Solution
|
- If degree of dissociation of HI is 0.1 then K(P) for reaction is: 2H...
Text Solution
|
- In the disociation of HI, 20 % HI is dissociated at equilibrium at a c...
Text Solution
|
- The equilibrium constant value K(p) for the equilibrium : H(2) (g) + I...
Text Solution
|
- At 500 K, equlibrium constant, K(c) for the following reaction is 5. ...
Text Solution
|