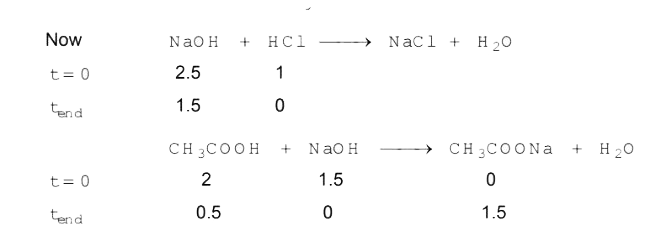
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 3 gram of acetic acid is mixed in 250 mL of 0.1 M HCl. This mixture is...
Text Solution
|
- What is the pH of solution in which 25.0 mL of 0.1 M NaOH is added to ...
Text Solution
|
- 40 ml of 0.1 M ammonia is mixed with 20 ml of 0.1 M HCI. What is the p...
Text Solution
|
- 40 ml of 0.1 M ammonia is mixed with 20 ml of 0.1 M HCl. What is the p...
Text Solution
|
- 3 gram of acetic acid is mixed in 250 mL of 0.1 M HCl. This mixture is...
Text Solution
|
- 20 mL 0.1 (N) acetic acid is mixed with 10 mL 0.1(N) NaOH solution. pH...
Text Solution
|
- When 20 mL 0.1 (N) CH3COOH and 10mL 0.1 (N) NaOH are mixed, the pH of ...
Text Solution
|
- PH of 0.1 M 500 ml CH3COOH+ 0.1M 500 ml NaOH solution (Pka CH3COOH= 4....
Text Solution
|
- PH of 0.1 M 500 ml CH3COOH+ 0.1M 250 ml NaOH solution (Pka CH3COOH= 4....
Text Solution
|