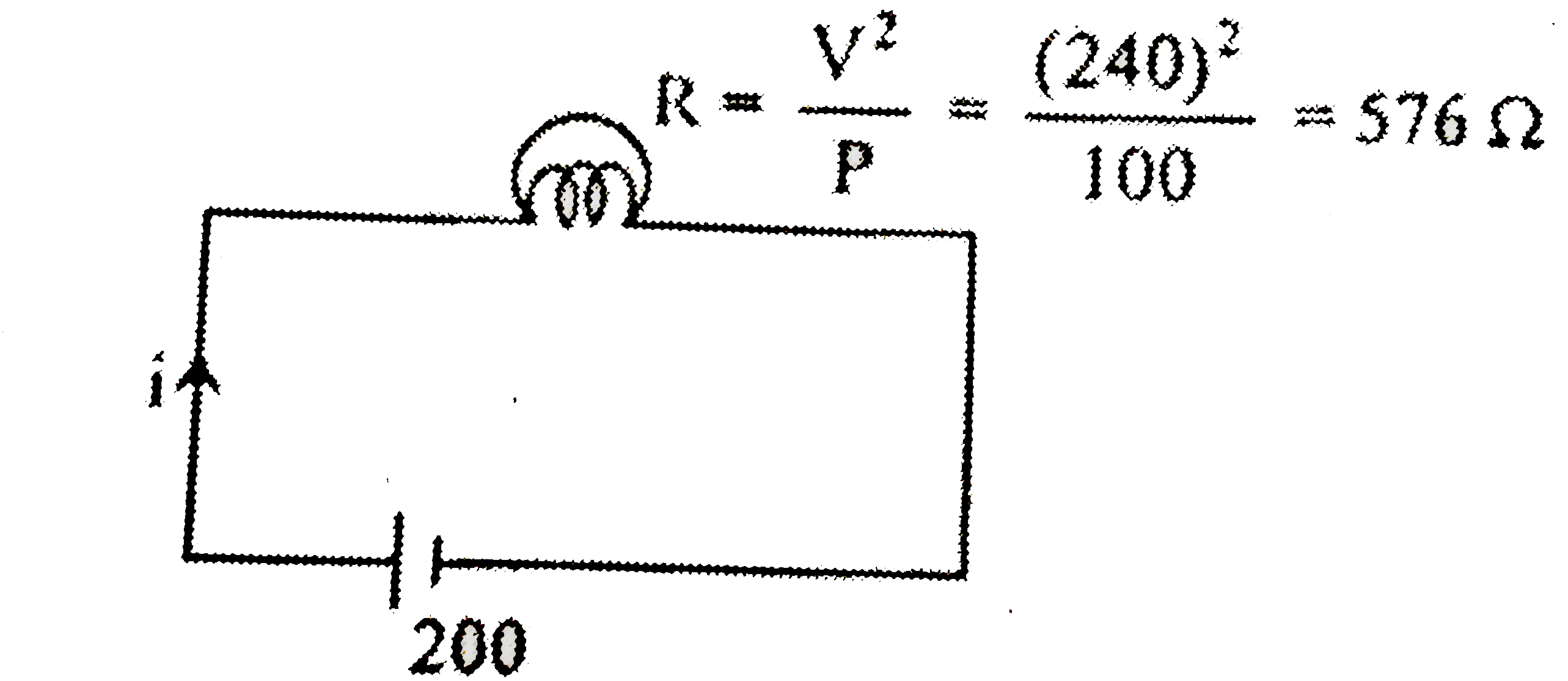A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CAREER POINT-MOCK TEST 2-PART (B)-CHEMISTRY
- When a 100 W, 240 V bulb is operated at 200 volt, the current in it is...
Text Solution
|
- Sodium thiosulphate, Na(2)S(2)O(3).5H(2)O is used in photography to
Text Solution
|
- The cyanide ion CN and N(2) are isoelectronic, but in contrast to CN^(...
Text Solution
|
- Ferric ion forms a prussian blue coloured solution with K(4)[Fe(CN)(6)...
Text Solution
|
- Under what conditoin of temperature and pressur the formation of atom...
Text Solution
|
- The reaction of (S)-2 bromobutane with OH^(-) to produce (R )-butane-2...
Text Solution
|
- 4 mole of a mixture of Mohr's salt and Fe(2)(SO(4))(3) requires 500mL...
Text Solution
|
- Total charge required for the oxidation of two moles Mn3O4 into MnO4^...
Text Solution
|
- Calculate standard free energy change for the reaction 2Ag+2H^(+)rarrH...
Text Solution
|
- Which one of the following ionic species will impart colour to an aque...
Text Solution
|
- Select incorrect order :
Text Solution
|
- In which of the following processes energy is absorbed ?
Text Solution
|
- An element (atomic mass = 100 g//mol) having bcc structure has unit ce...
Text Solution
|
- Select the correct statement -
Text Solution
|
- Element X crystallizes in 12 co - ordination fcc lattice. On applyng h...
Text Solution
|
- Which of the following is correct option for the free expansion of an ...
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- On increasing the temperature , the rate of a reaction:
Text Solution
|
- The molality of 15 % by wt solution of H2SO4 is
Text Solution
|
- Rate of reaction (r ) is plotted against temperature (T) for an enzyme...
Text Solution
|
- What volume of 75% alcohol by weight (d-0.80g//cm^(3)) must be used to...
Text Solution
|