A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CAREER POINT-UNIT TEST 10-PHYSICS
- If the wavelength of photon emitted due to transition of electron from...
Text Solution
|
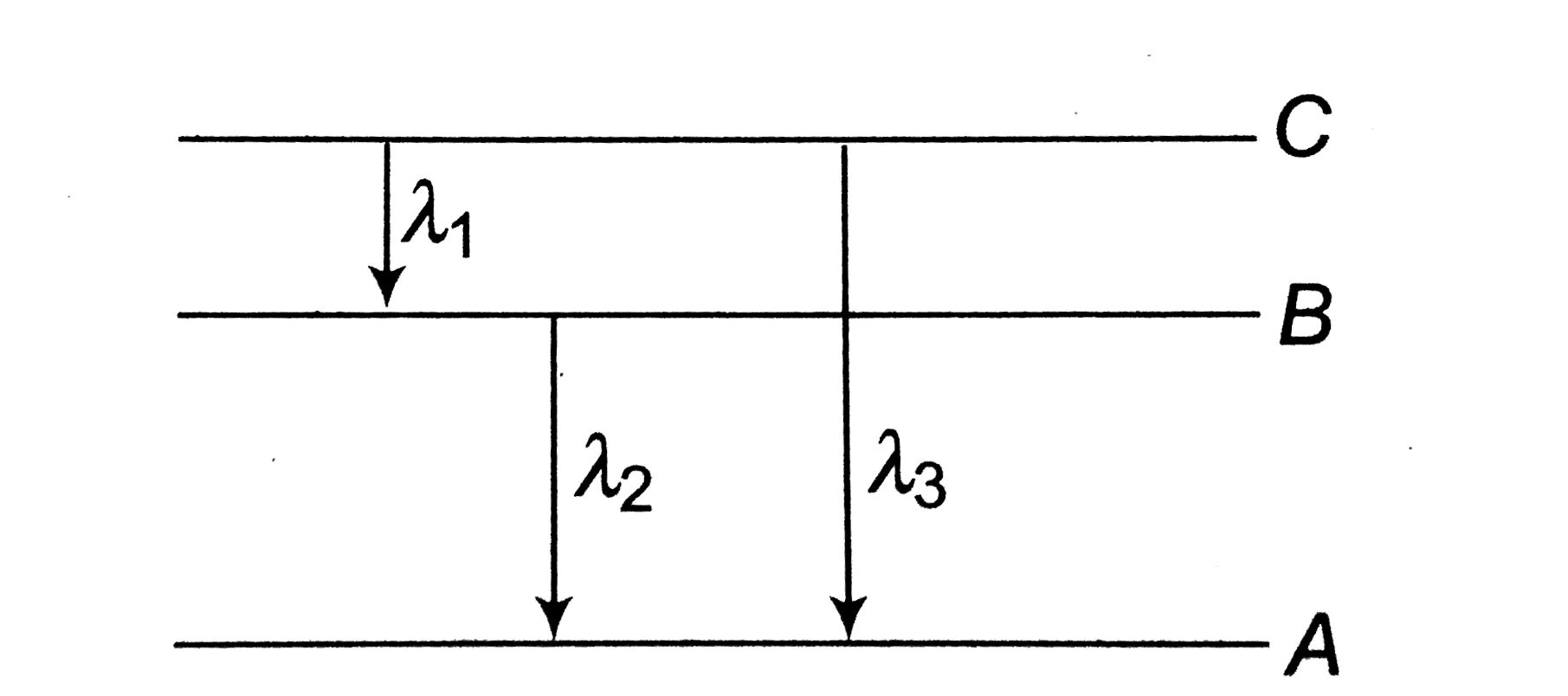
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- If, in a hydrogen atom, radius of nth Bohr orbit is r(n) frequency of ...
Text Solution
|
- A particle of mass m moves along a circular orbit in centrosymmetrical...
Text Solution
|
- In rutherford's experiment, the mumber of alpha-particles scattered th...
Text Solution
|
- A Hydrogen atom and Li^(++) ion are both in the second excited state....
Text Solution
|
- An e-m wave of wavelength lambda is incident on a photo sensitive surf...
Text Solution
|
- A 100 W light bulb is placed at the centre of a spherical chamber of r...
Text Solution
|
- In Davisson-Germer experiment, the correct relation between angle of d...
Text Solution
|
- The longest wavelength that can be analysed by a sodium chloride cryst...
Text Solution
|
- Identify the graph which correctly represent the Moseley's law-
Text Solution
|
- In X-ray tube , when the accelerating voltage V is halved, the differe...
Text Solution
|
- Light of wavelength lamda strikes a photoelectric surface and electron...
Text Solution
|
- Photoelectric emission is observed from a metallic surface for frequen...
Text Solution
|
- If K(1) and K(2) are maximum kinetic energies of photoelectrons emitte...
Text Solution
|
- Given that a photon of light of wavelength 10,000 angstrom has an ener...
Text Solution
|
- In an alpha -decay, the kinetic energy of alpha-particles is 48 MeV a...
Text Solution
|
- Carbon -14 decays with half-life of about 5, 800 years. In a sample of...
Text Solution
|
- A radioactive element decays by beta-emission. A detector records n be...
Text Solution
|
- Find the Q value of the reaction P + .^(7) Li rarr .^(4) He +.^(4) He....
Text Solution
|