Text Solution
Verified by Experts
Topper's Solved these Questions
THE S-BLOCK ELEMENTS
NCERT EXEMPLAR|Exercise Matching The Columns|3 VideosTHE S-BLOCK ELEMENTS
NCERT EXEMPLAR|Exercise Assertion and Reason|2 VideosTHE S-BLOCK ELEMENTS
NCERT EXEMPLAR|Exercise Long Answer Type Questions|8 VideosTHE P-BLOCK ELEMENTS
NCERT EXEMPLAR|Exercise Long Answer|10 VideosTHERMODYNAMICS
NCERT EXEMPLAR|Exercise Multiple choice questions|62 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-THE S-BLOCK ELEMENTS -Short Answer Type Questions
- When heated in air, the alkali metals form various oxides. Mention the...
Text Solution
|
- Complete the following reactions (i) O(2)^(2-)+H(2)O rarr (ii) O(2)^...
Text Solution
|
- Lithium resembles magnesium in some of its properties. Mention two suc...
Text Solution
|
- Name an element from group 2 which forms an amphoteric oxide and a wat...
Text Solution
|
- Discuss the trend of the following (i) Thermal stability of carbonat...
Text Solution
|
- Why are BeSO(4) and MgSO(4) readily soluble in water while CaSO(4),SrS...
Text Solution
|
- All compounds of alkali metals are easily soluble in water but lithium...
Text Solution
|
- In the Solvay process, can we obtain sodium carbonate directly by trea...
Text Solution
|
- Write Lewis structure of O(2)^(-) ion and find out oxidation state of ...
Text Solution
|
- Be and Mg atoms do not impart colour to the flame.
Text Solution
|
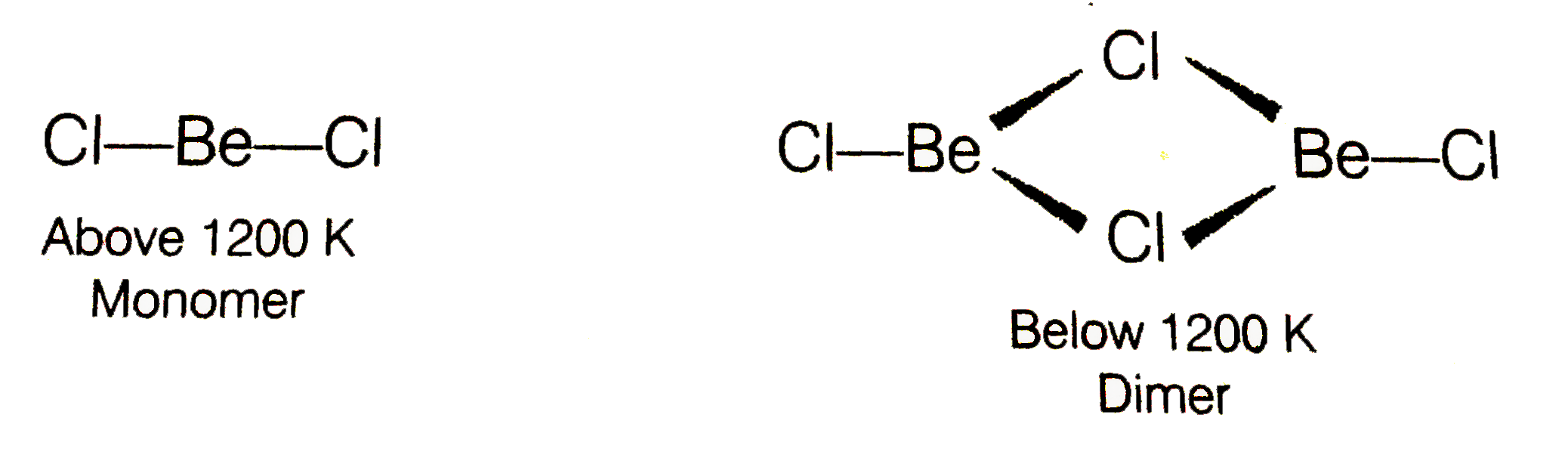
- What is the structure of BeCI(2) molecule in gaseous and solid state?
Text Solution
|
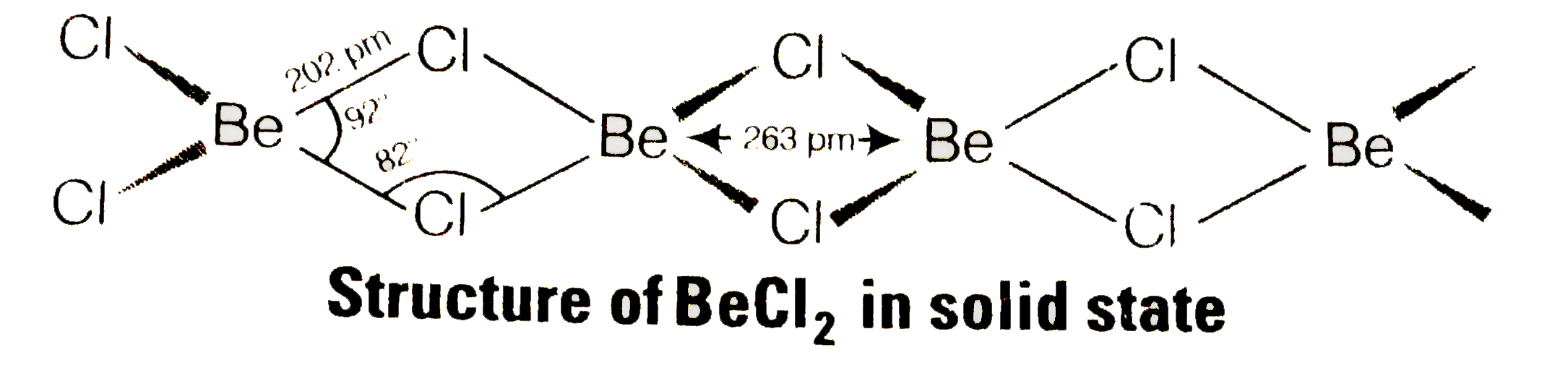 ltbr. In vapour state, above 1200K, it exists as a monomer having linear structure and zero dipole moment. But below 1200K, it exists as dimer structure even in vapour state.
ltbr. In vapour state, above 1200K, it exists as a monomer having linear structure and zero dipole moment. But below 1200K, it exists as dimer structure even in vapour state.