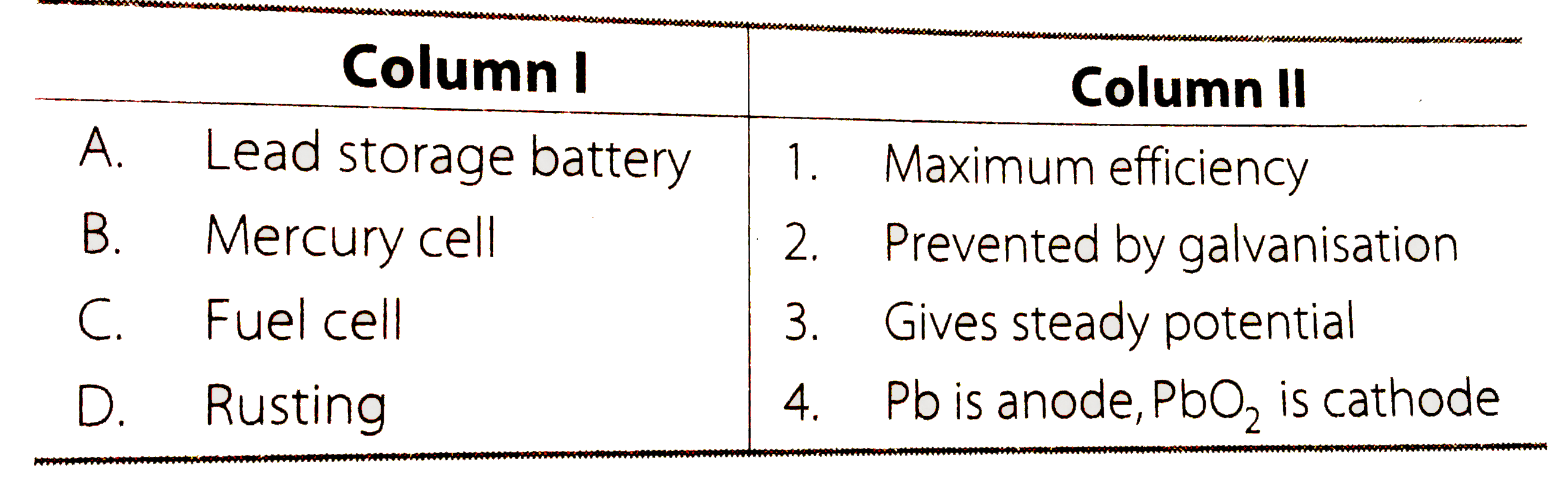Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ELECTROCHEMISTRY-Electrochemistry
- Why on dilution the Lambda(m) of CH(3)COOH increases drastically, whil...
Text Solution
|
- Match the terms given in column I with the units given in column II.
Text Solution
|
- Match the terms given in Column I with the items given in Column II. (...
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II on the basis of data given b...
Text Solution
|
- Assertion(A) Cu is less reactive than hydrogen. Reason(R) E(Cu^(2+)/...
Text Solution
|
- Assertion (A) E("cell") should have a positive value for the cell to f...
Text Solution
|
- Assertion (A) Conductivity of all electrolytes decreases on dilution. ...
Text Solution
|
- Assertion(A) Lambda(m) for weak electrolytes shows a sharp increase wh...
Text Solution
|
- STATEMENT-1: The voltage of mercury cell remains constant for longer p...
Text Solution
|
- Assertion (A): Electrolysis of NaCl solution gives chlorine at anode i...
Text Solution
|
- Assertion : For measuring resistance of an ionic solution an AC so...
Text Solution
|
- Assertion : Current stops flowing when E(cell)=0. Reason : ...
Text Solution
|
- Assertion (A): E(Ag^(+)//Ag) increases with increase in concentration ...
Text Solution
|
- Assertion (A) Copper sulphate can be stored in zinc vessel. Reason(R...
Text Solution
|
- Consider the figure and answer the following question. (i) Cell '...
Text Solution
|
- Consider figure from the above question and answer the questions (i) t...
Text Solution
|
- What is the relationship between Gibbs free energy of the cell reactio...
Text Solution
|