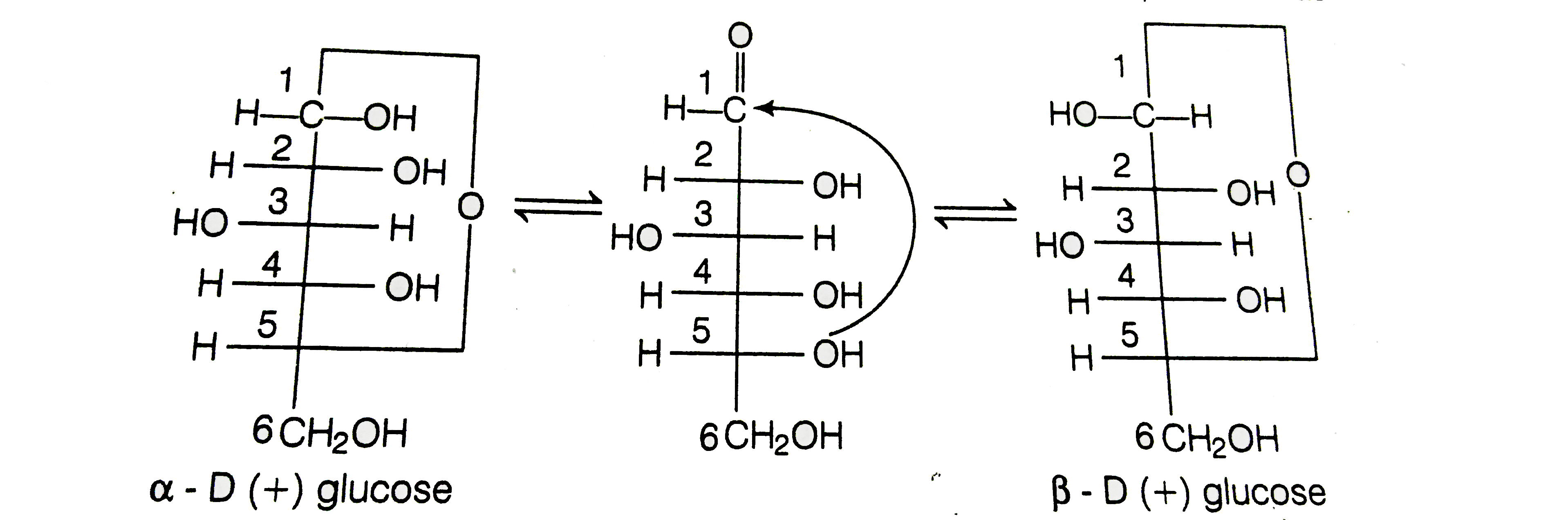
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-BIOMOLECULES -Biomolecules
- What are glucosidic linkages? In which type of biomolecules are they p...
Text Solution
|
- Which monosaccharide units are present in strach, cellulose and glucos...
Text Solution
|
- How do enzymes help a substrate to be attacked by the reagent effectiv...
Text Solution
|
- Descrive the term D- and L-configuration used for amino acids with exm...
Text Solution
|
- How will you distinguish 1^(@) and 2^(@) hydroxyl groups present in gl...
Text Solution
|
- Coagulation of egg white on boiling is an example of denaturation of p...
Text Solution
|
- Match the vitamins given in Column I with the deficiency disease they ...
Text Solution
|
- Match the following enzymes given in Column I with the reactions they ...
Text Solution
|
- Assertion (A) D(+)- Glucose is dextrorotatory in nature. Reason (R) ...
Text Solution
|
- Assertion (A) Vitamin D can be stored in our body. Reason (R) Vitami...
Text Solution
|
- Assertion (A) beta-glycosidic linkage is present in maltose. Reason ...
Text Solution
|
- Assertion (A) All naturally occurring alpha-aminoacids except glycine ...
Text Solution
|
- Assertion (A) Deoxyribose, C(5)H(10)O(4) is not a carbohydrate. Reas...
Text Solution
|
- Assertion (A) Glycine must be taken through diet. Reason (R) It is a...
Text Solution
|
- Assertion (A) In presence of enzyme, substrate molecule can be attacke...
Text Solution
|
- Write the reactions of D-glucose which can't be explained by its open ...
Text Solution
|
- On the basis of which evidences D-glucose was assigned the following s...
Text Solution
|
- Carbohydrates are essential for life in both plants and animals. Name ...
Text Solution
|
- Explain the terms primary and secondary structures of proteins. What i...
Text Solution
|
- Write the structures of fragments produced on complete hydrolysis of D...
Text Solution
|